How and why Technokontrol works
How and why Technokontrol works
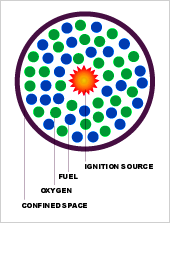
Why does an explosion occur? In order for a deflagration explosion to occur four elements are required. These are; Fuel, Oxygen, An ignition source and a confined space.
The element of "Fuel" can come from a bulk material that produces dust clouds, a flammable gas or a volatile chemical that creates vapours. The element of “Oxygen” is readily available in almost all plant processes. The source of “ignition” element may be generated by a fire, a flame, a welding arc, a spontaneous combustion, frictional sparks or electrostatic hazards. Finally practically all the plant processes provide the element of “enclosed volumes”. Once the above four elements are together, the potential for an explosion exists.
How does an explosion develop?

Technically an explosion is a freely propagating combustion wave, or deflagration, moving at less than the speed of sound. Unconfined, this flame front travels initially at slow speeds, but normally the flame increases in velocity shortly after ignition to form a high pressure wave.
As most industrial processes usually are not designed to withstand such a wave, the pressure develops into an explosion, a rupture occurs releasing a destructive pressure shock wave and flame.
What is B.L.E.V.E. explosion?
TechnoKontrol works exclusively mechanically and without altering the chemical properties of the fuel.
Its geometry in form of polyhedral mesh fulfils the following assignments:
- The kinetic energy of gases is restrained in its radial advance from the starting point of inflammation. So the progression in spherical layers (known in the technical field as “onion layers”) is destroyed.
- The calorific energy that contributes to the chain reaction is cushioned by the great heat absorption that offers the surface of the mesh.

In order to obtain this effect we need to ensure that the relation of increased surface, by volume treated [in the order of 3000 centimetres square per litre] as well as the minimum thickness of the mesh settling down in 0.06 mm.
The surface of the material determines the speed of absorption of calories but it is also necessary to ensure that the material has the sufficient volume, so that the heat capacity is enough to admit the initial calories (Energy of Activation) and the subsequent chain reaction is avoided. Actually these objectives are obtained by giving a thickness to the raw material of 0.06 mm.
It is important to emphasize that as previously described this is only valid for the initial moment where the objective is to avoid the chain reaction. Just after the inflammation, the material and the fuel equals the temperature to ensure that the gradient becomes zero and therefore, there is no longer a heat accumulation; the gas locked up in the deposit moves away from the stoichiometric mixture and when the air becomes scarce, the danger of an explosion completely disappears.
It arrives then, to a permanent regime in which the product works in analogous form similar to the one of a “wick”, which allows that the gas only becomes inflamed in the fuel filling mouths, as well as in the possible fissures that could cause a hypothetical accident or shock, overpressure, fatigue or overheat. As the gas is consumed in its combustion, the liquid evaporates and the gas is consumed until it reaches total extinction.
In addition to the above, the following benefits may be added:
- Due to the potential of oxidation of the aluminium mesh of 1.66 volts, opposed to 0.763 volts of zinc, this material becomes a very effective anode protection that guarantees no electro corrosion of the deposit.
- Then the mesh constitutes in itself a set of screens that helps to avoid the fast movements of the liquids, water hammers (violent movements of the liquid in the deposit) and also the accumulation of static loads, thus being able to therefore recalculate the thicknesses, as much as, the corresponding reinforcements in tanks with the consequent reduction in price of cost in its construction.
- Reduces the evaporation in the fuel tanks as normally when the fuel within the tank evaporates it produces a change of state which in turn generates a decrease of temperature within the boundary layer. This decrease of temperature is lead quickly from the surface of the liquid, through to the alloy of the Aluminium mesh which with its great heat conductivity (208 Watios/m ºC) means that the steam is condensed even further thus helping to reduce evaporation of the most volatile but powerful components and also the most poisonous of organic fuels.
TechnoKontrol Alloy Characteristics
- How it Works
- Physical Characteristics
- Technical Characteristics
- Flammable & Combustible Liquids - Hazards
- What is B.L.E.V.E. Explosion?
- TK-Global Engineering
- TK-Global Electronics
- TK-Global Environment
- TK-Global Technotelecom
- TK-QSSHE
- TK-Financing
- TK-Emergency Call Center
TechnoKontrol product and application videos
- TK Outdoor Tests Presentation - Fire Services / KNPC Kuwait
- Demonstration video
- Liquids balance
- Gas cylinders manufacturation process
- Panel at 1600°C 2 hours
- Wall at 1600°C +2 hours
TechnoKontrol Wikipedia

TechnoKontrol: 1st in Spain for own Technology patents 2013 & 2014

- Ministerio de Industria Turismo y Comercio-OEPM
Technokontrol, the number 1 corporation in Spain filing for its own technology patents, trademarks and utility patents (SPA) - OEPM 2013 The year in numbers (SPA)
TechnoKontrol has been fully verified and certified by Bureau Veritas

Certificates
- ISO 9001 - ES108784-1
- ISO 14001 - ES108782-1
- ISO 45001 - ES108783-1
- ISO 4126
- ISO 28000
- ISO 37000

NFPA-National Fire Protection Association
NFPA-Asociación Nacional de Protección contra el fuego
TechnoKontrol is a member of the NFPA

NFPA 69: Standard on Explosion Prevention Systems, 2016 Edition
Prevent deflagration explosions due to combustible dust particles, gases or vapors with NFPA 69. Combustible dust, gases and vapors produced in industrial settings can pose a significant safety hazard.NFPA 69: Standard on Explosion Prevention Systems offers definitive guidance on explosion protection and prevention systems.
ATEX - European Antiexplosive Safety Directives

- ATEX Guide: Protection in explosive atmospheres
- ATEX-EU/HAZLOZ-USA/NFPA-USA/DSEAR-UK/Explosion Directives TechnoKontrol Additional Anti-Explosion Data
- ATEX Directive in EU Directives
Dangerous Substances and Explosive Atmospheres Regulations - United Kingdom/ATEX

United Nations Economic Commission for Europe - UNECE - TechnoKontrol

- TechnoKontrol information for the UNECE BLEVE working group
Proposal transmitted by the governments of Spain and France
The European Parliament and The Council

Technokontrol's Products & Services are insured by

TK-Global Engineering - Where efficiency and reliability become a reality


More info
Should you want to receive more information, please contact us.
